FISH analysis for Acute Myeloid Leukaemia
| Category | Specialist Integrated Haematological Malignancy Diagnostic Service (SIHMDS) >> Cytogenetics |
|---|---|
| Test background |
FISH involves the application of fluorescent DNA probes specific to genes or genetic regions of interest that highlight abnormalities involving these regions. |
| Clinical Indications |
Acute myeloid leukaemia (AML) is the most common acute leukaemia in adults and recurrent genomic findings define the classification of AML. Cytogenetic and FISH testing is delivered in line with the National Genomic Test Directory (NGTD) for Cancer. A rapid FISH panel is performed on all diagnostic AML cases and is reported within 3 calendar days. 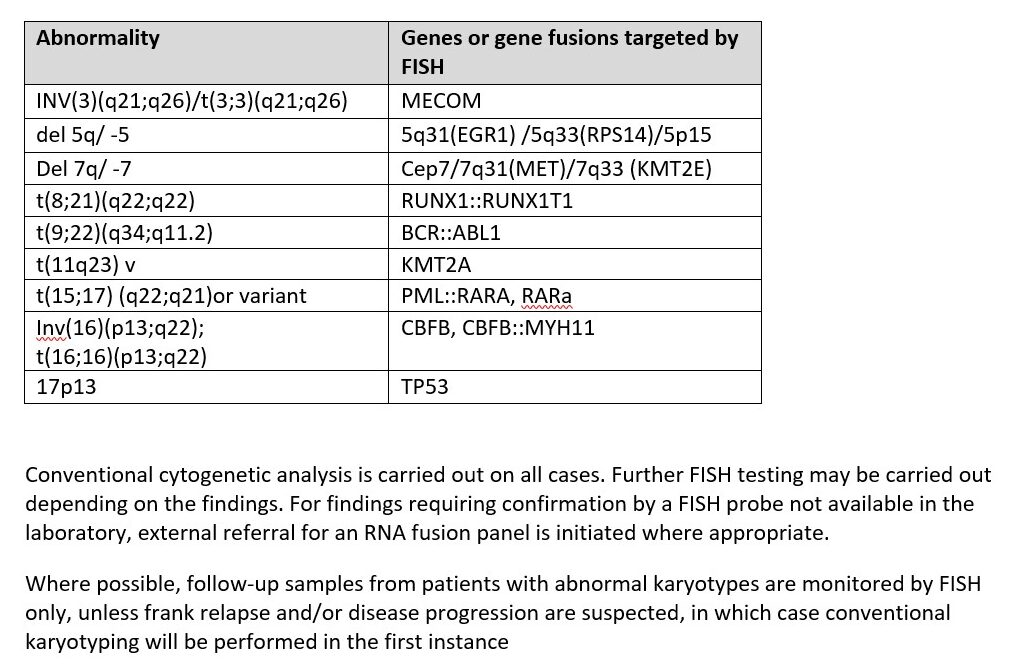
|
| Reference range | N/A |
| Sample & container required | A minimum of 2-3mls Bone marrow in lithium heparin. 3-5mls peripheral blood in lithium heparin (if disease cells are present in sufficient numbers to allow cell culture and/or FISH studies, as appropriate) EDTA PB and BM samples (>1ml) are acceptable for FISH only studies as appropriate. Please note the laboratory does not provide transport medium. Samples sent in transport media from an external laboratory containing Lithium heparin will be accepted if no other media available |
| Sample volume | Samples would not be rejected on the basis of small volume, however, 5 mL is ideal. |
| Turnaround time | Rapid AML FISH PANEL tests 95% should be reported within 3 calendar days Urgent diagnostic or relapse karyotype: 95% should be reported within 14 calendar days Post treatment follow-up samples are treated as routine; 95% should be reported within 21 calendar days |
| Notes | If the blood counts are abnormal (high or low white cell count) the volumes of BM/PB requested can be adjusted accordingly. For culture (karyotyping) a WCC of 5×10^6 cells per ml is optimal. Delayed samples: Samples should arrive in the laboratory as soon as possible after sampling. Samples delayed in transit may yield poor quality or failed results. |
